eyeforpharma Philadelphia 2014
Make customer centricity work: smart pharma mindsets, models and technology that will seal commercial success
J&J Recalls Glucose Meters After Patient Dies
Johnson and Johnson recalls their OneTouch Verio line of blood glucose meters, after the death of a patient.
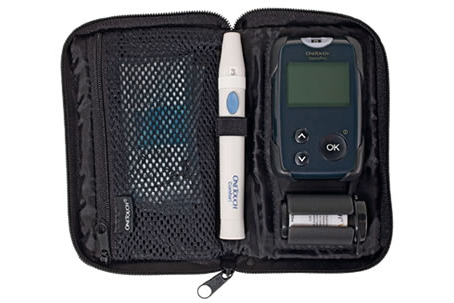
J&J’s Lifescan unit revealed in a statementthat its blood glucose meters were giving inaccurate readings, or shutting down completely, once blood sugar level rose beyond a certain level; however it stopped short of attributing the patient’s death to this malfunction, saying instead that it “has not determined whether the OneTouch® Verio®Pro Meter was a causal factor.” The incident was first reported to have been a “serious adverse event,” only later being acknowledged as a patient death in a statement by a spokesman to Bloomberg news.
As the Lifescan statement explains, “The likelihood of experiencing an extremely high blood glucose level of 1024 mg/dL or higher is remote; however, when such a blood glucose level occurs, it is a serious health risk requiring immediate medical attention. Because these products do not provide an appropriate warning at glucose levels of 1024 mg/dL or higher, diagnosis and treatment of extreme hyperglycemia may be delayed or incorrect treatment may be given resulting in potentially serious health risk or fatality.” Almost 1.9 million blood glucose meters are affected by the recall, including 90,000 in the U.S; Ernie Knewitz, a J&J spokesman, described sales of the Verio systems as “very modest,” although Lifescan’s diabetes care range overall generated $2.6 billion in sales last year.
Since 2010 J&J has had to recall a series of products, including some well-known OTC brands such as Tylenol, Motrin, Rolaids, Sudafed and Benadryl, as well as Acuvue contact lenses, K-Y Jelly, syringes, hip replacement devices and the Topamax epilepsy med. J&J’s Ethicon unit has suspended its marketing of four vaginal mesh implants after being sued by around 1,800 women who claim the products caused them serious internal injuries.
Due to the spate of recalls, J&J is now operating under a consent decree regarding three of its manufacturing plants, meaning that they will undergo a full refit and will be under the watchful eye of the FDA from now on. While investors seem to have agreed to overlook J&J’s recent troubles, sending company stock climbing 30 per cent over the past year, the firm still have a long way to go to convince consumers and physicians that they have sorted out their manufacturing issues– making this latest setback all the more unfortunate.
eyeforpharma Philadelphia 2014
Make customer centricity work: smart pharma mindsets, models and technology that will seal commercial success
