Experts Call on FDA for Changes To “Unsafe” Medical Device Approvals
An academic perspective published in the New England Journal of Medicine makes an urgent call to the FDA to prevent unsafe medical devices from entering the marketplace.
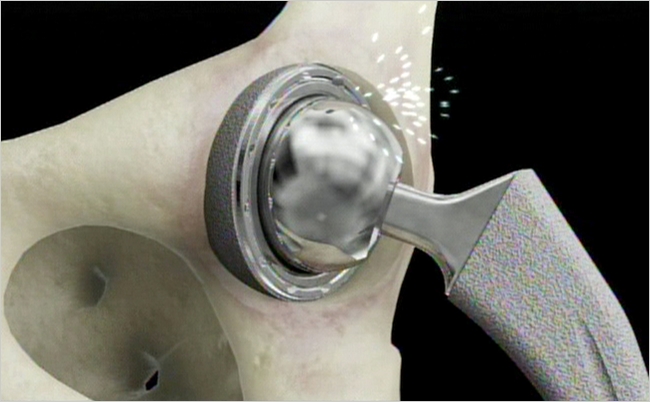
Authors of this paper from the USCF Women’s Cardiovascular Services and the Australian Joint Registry state that some ‘high risk’ surgical devices were approved by the FDA without being tested in clinical trials, which would determine the safety and effectiveness of a product. The paper looks into the complex history of how metal-on-metal hip implants entered the marketplace. These implants constantly failed at a very high rate that often required repetitive surgery about four times as much as a traditional hip replacement.
According to the US Centres for Disease Control and Prevention, national hospitals perform 48 million medical procedures every year. Of this figure, around 676,000 patients undertake total knee replacement surgeries and 327,000 get total hip replacement surgeries. The authors of the paper seek changes in the approval process of the FDA for metal-on-metal hip replacement services as well as other high risk devices for the medical device marketplace.
Redberg, who is the director of the UCSF Women's Cardiovascular Services, says: “This could be potentially very dangerous. Many Americans – patients and even physicians - are not aware of how many devices in this country are on the market without having clinical data of safety and effectiveness. If those hip implants are recalled, besides the problem of having to remove them because they're very painful, they can release chromium ions into the blood stream which pose an unknown risk. Patients would also undergo significant disability having a second, third or fourth hip operation.”
She added: “Some patients' mobility will decline to the point of needing walkers or wheelchairs to get around and other serious events up to and including death can occur from subsequent operations. And that's just for the metal-on-metal implants.”
At present, FDA loopholes in the 510(k) clearance allow these medical devices to avoid clinical trials as a result of the ‘substantial equivalence’ condition. So, medical devices that are similar to those already approved or fall under ‘predicate devices’ will bypass clinical testing. Predicates include voluntary recalled devices under the 510(k) clearance if FDA did not want their removal from the market or a court did not discover that they were misbranded or misrepresented in any way.
Redberg says: “High-risk medical devices should go through randomized clinical trials done in people so we can assure patients they are safe and effective. Even the more stringent pre-market approval (PMA) process doesn't always mean that you actually have gone through randomized clinical trials, so we have to make sure these devices not only go through pre-market approval but randomized clinical trials as well.”
She also offers advice to patients undergoing a medical procedure: “Just like for any kind of procedure, I would ask, 'Are there scientific data that show this procedure or device is going to help me? Has it been studied in clinical trials? Has it been studied in patients like me? What are the risks? What are the alternatives?”
While technological advancements in medicine can offer better health outcomes for patients, regulators should always test their safety and efficiency; even it has some similarities to an already approved device.

